The approval of epidyolex for the treatment of refractory pediatric epilepsy is providing an increasing body of evidence on the therapeutic potential of cannabidiol (CBD), which had already showed promise to address other clinical conditions such as anxiety, psychotic symptoms, or substance use disorders. Owing to this therapeutical promise and fueled by media hype, the popularity of CBD has translated into an exponential surge both in consumer’s interest and availability of artisanal (slash illegal) CBD products in several countries, including the UK, where it is estimated that 8-11% of adults had tried CBD by June 2019.
However, clinical research with CBD is usually conducted with much higher oral doses (300-900 mg) than those achieved, and generally recommended, when using artisanal CBD preparations (20-50 mg). What sense can we then make out of this oral, low-dose consumption of CBD? Here are 3 recent publications to shed some light on this dilemma:
- In a recent review, Chestney and colleagues from King’s College London concluded there is little evidence that artisanal CBD products have “significant pharmacological activity or provide health benefits”. A compelling argument is that low oral doses are extremely unlikely to produce plasma concentrations of CBD that can result effective at any of its putative molecular targets, in particular serotonin 5-HT1A receptors.
- Earlier this year, Julie Moltke and Chadni Hindocha published the results of a cross-sectional, retrospective study based in an anonymous online questionnaire. A majority of the sample surveyed in this study (387 UK users) reported that CBD helped with their symptoms (sleep disturbances, stress, and anxiety) and they often used doses below 50 mg (see Fig. 1).
- In a study published a few weeks ago, researchers from Syracuse University reported the significant impact that analgesic expectancies had on the ability of CBD at reducing human pain reactivity (pain threshold, tolerance, intensity, and unpleasantness). Specifically, authors found that CBD-induced reductions in pain unpleasantness were caused by both psychological expectancies for receiving a CBD analgesic and administration of CBD, which resonates with the increasing evidence pointing at a relevant role of the endocannabinoid system in modulating the placebo effect.
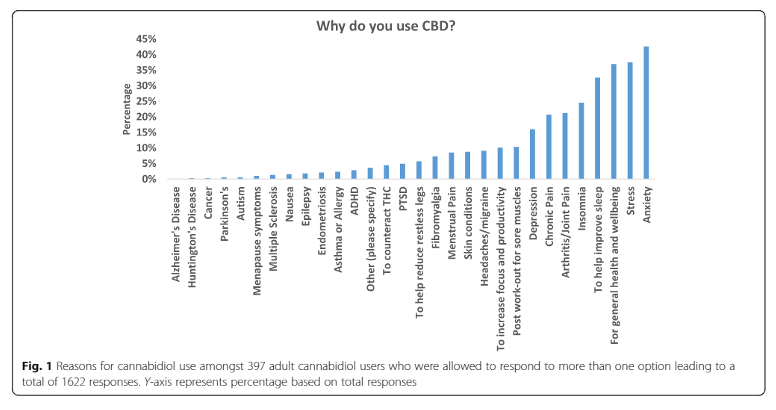
All three studies conclude that further research is needed to better understand the efficacy and safety of low dose oral preparations of CBD. Tomorrow, June 8th, Khiron will be hosting together with the Medical Cannabis Clinicians Society, a webinar to provide a comprehensive summary of the state of the evidence on the therapeutic potential of CBD-rich, cannabis-based medicinal products as well as valuable insights from leading experts in the medical field. Please register in the link below:
Clinical experience with CBD: dried flower and oral extracts
- Chesney, E., McGuire, P., Freeman, T. P., Strang, J. & Englund, A. Lack of evidence for the effectiveness or safety of over-the-counter cannabidiol products. Ther. Adv. Psychopharmacol. 10, 204512532095499 (2020).
- Moltke, J. & Hindocha, C. Reasons for cannabidiol use: a cross-sectional study of CBD users, focusing on self-perceived stress, anxiety, and sleep problems. doi:10.1186/s42238-021-00061-5.
- De Vita, M. J. et al. The effects of cannabidiol and analgesic expectancies on experimental pain reactivity in healthy adults: A balanced placebo design trial. Exp. Clin. Psychopharmacol. (2021) doi:10.1037/pha0000465.

